Synthesis of Aspirin Introduction: Most drugs are chemical compounds which are described as "organic compounds" because they ...
Most drugs are chemical compounds which are described as "organic compounds" because they are comprised primarily of the elements carbon, hydrogen and oxygen. The present experiment will be the synthesis of a familiar organic compound called aspirin. The common chemical name is acetylsalicylic acid.
Aspirin, the most widely used drug in the world, has an interesting history. Nearly 2500 years ago, Greeks reported that extracts of willow and poplar bark could be used to relieve pain and symptoms of illness. There are reports that American Indians, before the time of Columbus, used special teas made from the bark of willow trees to reduce fever. In 1763, the Reverend Edward Stone introduced these extracts and teas to the Europeans and in the early 1800's the active ingredient in willow bark (and in the flowers of the meadow sweet plant which had similar therapeutic characteristics) was isolated and identified as salicylic acid (from salix, the Latin name for the willow tree).
Although salicylic acid could be synthesized in the laboratory and large quantities became available for therapeutic use in the mid 1800s, the compound's acidic properties caused irritation in the moist membranes of the mouth, throat and stomach. In 1875, the sodium salt was introduced and although the salt was less sour to the taste (actually it had an objectionable sweetish taste), it did not alleviate the gastric discomfort problems.
Acetylsalicylic acid, a weaker acid than salicylic acid, was found to have the medicinal properties of salicylic acid without having the objectionable taste or producing the stomach problems. The acetyl group effectively masks the acidity of the drug during its ingestion and after it passes into the small intestine, it is converted back to salicylic acid where it can enter the bloodstream and do its pain relieving action. Bayer called its new product "aspirin," the name being derived from "a" for acetyl, and the root "-spir", from the Latin name Spiraea ulmaria, the meadow sweet flower, from which salicylic acid had been isolated. It was patented by Bayer in 1899, and in 1915 Bayer Aspirin tablets became commercially available as a non- prescription drug. The trademark is still owned by the Bayer A.G. company in Germany.
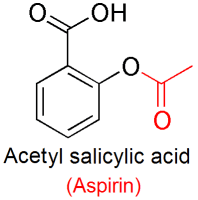
Salicylic acid reacts with acetic anhydride according to the following reaction:
Notice that this reaction does not contain a single reaction arrow, but a double arrow. Up to this point, we have assumed that all reactants only produce products. However, as the reactants are used up, their concentration goes down, while the concentration of the products increases. It turns out that the products can then react and produce the original reactants. Because of this, it will be very difficult to obtain a 100% yield of the desired product. This is known as an equilibrium reaction and there are several things we can do to help increase the yield of final product, but that will be the focus of most of our work in Gen Chem II.
The above reaction is an example of an organic synthesis called esterification. Esterification is the acid catalyzed reaction of a carboxyl (-COOH) group and an -OH group of an alcohol or phenol to form a carboxylate ester. In the synthesis of aspirin the -OH group is the phenolic -OH group attached to ring of the salicylic acid. The acetyl group, -COCH3 comes from acetic anhydride, and the reaction is catalyzed by phosphoric acid, H3PO4.
Procedure: Synthesis of Aspirin:
- This lab is very time intensive and you must 'multitask' if you are going to finish. The actual reaction to produce the aspirin only takes a minute or two, however, there is a lot of preparation before you are ready to run the reaction. It is important to study the procedure before coming to lab and not just 'cookbook' it.
- Add approximately 200 mL of distilled water to your 600 mL beaker and heat it with your hot plate until it boils.
- Construct an ice bath by filling your 450 mL beaker half way with ice and put in just enough distilled water to cover the ice. Now put 50-75 mL of distilled water into a clean 150 mL beaker and place that into the ice bath. This will cool the water so it will not dissolve your aspirin when you quantitatively transfer it from the Erlenmeyer flask to the Buchner filter.
- You will need to thoroughly clean a 125 mL Erlenmeyer flask and your largest test tube with soap and water. Rinse them with distilled water and use a paper towel to remove all visible signs of water. Use your Bunsen burner (outer blue cone) to flame dry the Erlenmeyer flask and the test tube.. BE CAREFUL NOT TO BURN YOURSELF. Be sure to let the flask and the test tube cool to room temperature and record their mass to the nearest 0.001 g.
- Weigh out approximately 1.5 g of salicylic acid (to the nearest 0.001 g) directly into a 125 mL Erlenmeyer flask. It is important that no solid remains on the sides of the flask, since any solid that is not in solution does not react and will decrease your yield.
- Take your Erlenmeyer flask to the hood and measure out 3 mL of acetic anhydride (~0.027 mole) with one of the 5 mL graduated cylinders. DANGER: ACETIC ANHYDRIDE IS EXTREMELY CAUSTIC. WEAR GLOVES WHEN POURING IT AND BE VERY SURE THAT IT DOES NOT GET ON YOUR HANDS. IF ACETIC ANHYDRIDE SHOULD COME IN CONTACT WITH THE SKIN, WASH IMMEDIATELY WITH PLENTY OF WATER FOR AT LEAST 5 (FIVE) MINUTES. DO NOT BREATHE THE VAPOR.
- Add the acetic anhydride to the salicylic acid in the Erlenmeyer flask in such a way that any salicylic acid clinging to the inside of the flask is rinsed down into the bottom. Swirl the flask gently.
- After all the acetic anhydride has been added, add 10 drops of 85% phosphoric acid (H3PO4 - serves as a catalyst) and gently swirl the mixture.
- Place the flask in the hot water bath for approximately 5 minutes, swirling occasionally. All of the solid salicylic acid should have reacted by this time, leaving a clear colorless solution of the final product and any excess acetic anhydride.
- While the reaction mixture is still hot, add 3 mL of distilled water. CAUTION: THE DECOMPOSITION OF THE EXCESS ACETIC ANHYDRIDE IS EXOTHERMIC. HOT VAPOR COULD BE EVOLVED AND SPATTERING MAY OCCUR. STAND BACK.
- When the decomposition is complete, add an additional 25 mL of distilled water. Use some of it to rinse down the inside of the flask. Swirl the flask to achieve thorough mixing.
- Add a handful or two of ice to your 600 mL beaker that contains the hot water. It is now an ice bath.
- Place the Erlenmeyer flask in this ice water bath and swirl occasionally. Allow the flask to stand in the ice bath until crystallization is complete (about 15 minutes, you may need to add more ice during this time).
- Filter the crude product using your Buchner filtration apparatus and wash the aspirin three times with ~15 mL portions of cold water (this will remove any impurities). After washing, pull air through the product to remove excess moisture. The longer you allow the product to air dry with the suction on, the better your yield will be.
- While the aspirin is air drying, clean and dry your watch glass. Put a distinguishing mark on it and weigh it (to the nearest 0.001 g).
- Transfer your product to your weighed watch glass and place in the oven (place it on one of the shelves, NOT on the bottom of the oven, at less than 90°C) for 10-15 minutes. Remove your watch glass from the oven and let it cool to room temperature. Weigh the watch glass and product. Carefully break up any clumps to facilitate drying and put the watch glass back in the oven for another 10 minutes. Remove it, let it cool, and weigh again. If the two weights agree to 0.01g or less, then your product is dry. If the two weights to not agree to 0.01g, then you will need to continue the heating, cooling, and weighing cycle until two consecutive weights are within 0.01g.
- Once your product is dry, quantitatively transfer it to the test tube you cleaned earlier and use a cork to seal it.
- Create a label with the following information:: Your Name, Date, Product Name, Actual Weight of Product in the test tube, Theoretical Yield, and % Yield.
- Affix the label to the corked test tube and turn it in to your instructor. You will be testing the purity of this product in next week's lab.
Calculation of Yield:
At this point, you ought to be able to calculate the theoretical yield of a reaction in your sleep! Start by converting your grams of salicylic acid into moles using its molecular weight, then use the stoichiometry of the balanced chemical reaction to covert your moles of salicylic acid into moles of acetylsalicyclic acid, and then finally use the molecular weight of acetylsalicyclic acid to convert the moles to grams. This is the theoretical amount of aspirin you should generate if everything went perfectly. Then the % yield is your actual yield divided by the theoretical yield, times 100: Source: http://www2.volstate.edu/chem/1110/Synthesis_of_Aspirin.htm


Aspirin the wonder drug of which if it is discovered today will be given by prescription only. It has been in use as a pharmaceutical agent for over 100 years. The molecular
ReplyDeletetarget for its antiinflammatory and analgesic action is the acetylation of cyclooxygenase isoform, COX-2,
that converts arachidonic acid to prostaglandin endoperoxides then to PGF2alfa, PgE1,Pge2 Txb2 etc. This results to Inflamation. But with irriversible action of asprin on cyclooxygenase, the inflamation will be inhibited
So Aspirin therapy inhibits prostaglandin biosynthesis; yet via
acetylation of cyclooxygenase 2 (COX-2) it leads to bioactive lipoxins epimeric at carbon
Also reduced dose which we call baby asprin can selectively inhibit thromboxane a2 dependent platelet inducer which can induce ciovascular problems. The problem now is the inhibition of Cox1 which in involved in the cytoprotective action on the gastointestinal linings Today we have Cytotec which is PgE1 analog used to treat the ulceration induced by Asprin. The problem, it has abonificient problems, so we must continue with research to develop a a better agent that is safe for all